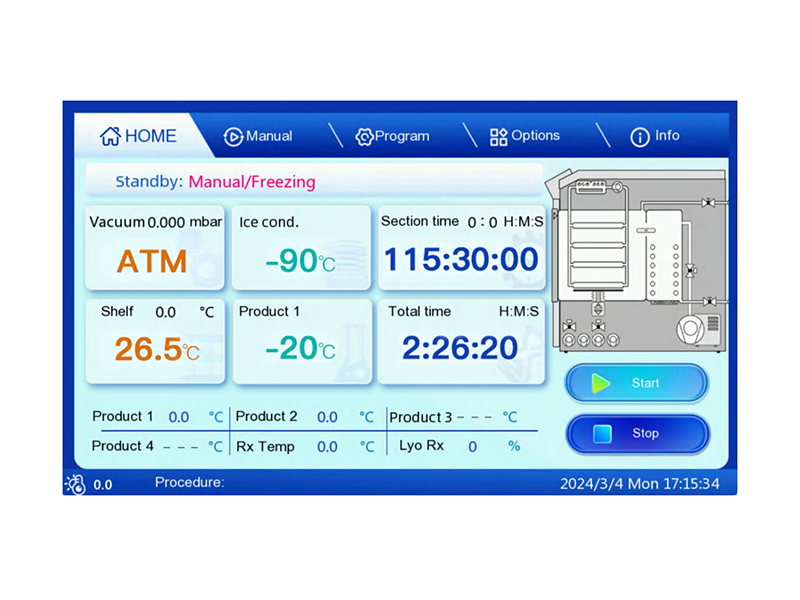
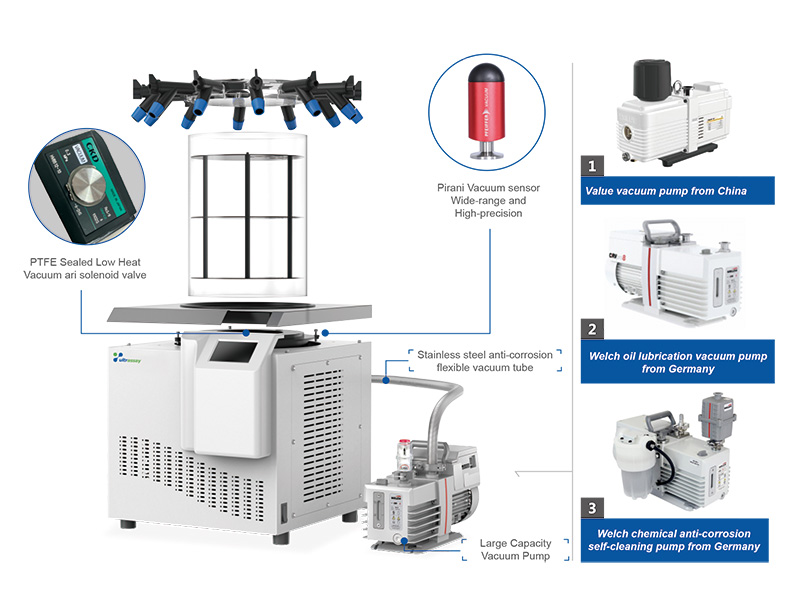
Ultrassay® pilot scale freeze dryer completely simulates the technical requirements of the industrial freeze dryer, with a compact and reasonable design, a large redundancy range, and conforms to GMP and FDA standards. The ice condensation capacity is 8~24kg, and the WRL controller and liquid temperature control shelf are adopted, so that the process of pre-freezing and freeze-drying can be precisely controlled on the main machine, and the process results are quite comparable with the production machine. In addition to supporting the eutectic point test system, pressure rise and pressure comparison method to determine the freeze-drying end point and freeze-drying curve recording software, hallan pilot -type freeze dryer can also be equipped with engineering WRL control software to directly Graphical computer control the freeze-drying process. It can be configured with H2O2 sterilization and high- pressure steam sterilization or integrated glove box system, which can be used for clean room operation through the wall. The cold trap and freeze-drying chamber are all designed with 316L stainless steel to ensure the cleanliness of the chamber and good ultimate vacuum and vacuum leakage rate to the greatest extent.
Ultrassay® pilot scale freeze dryer can be used for freeze drying of bacteria, viruses, plasma, serum components, antibodies, serum and vaccines, pharmaceutical products such as chloramphenicol, streptomycin, vitamins, enzymes, plant extracts for biochemical experiments, etc. and research and development.